Pharmaceutical Supply Chain Traceability
Collecting and contextualizing data from “Lab to Jab“ will improve yield, throughput, safety, and compliance
Frequently Asked Questions on Pharmaceutical Supply Chain Traceability
Over the last ten-years, technologies specific to software tools, has rapidly improved R&D, testing and manufacturing processes of pharmaceutical goods. This was clear during our current ongoing pandemic. Real-time information is more critical than ever before, all being mandated both from the FDA, Pharmaceutical OEM’s and now further heightened by the consumers. There is no argument we have seen a significant shift in the way products are being manufactured and sold by the innovative Pharmaceutical industry. The therapeutic competition globally, has a greater prevalence of large molecule drugs impacted in todays, biopharmaceutical expansion in the number of personalized or targeted products, and a rise of treatments for many orphan diseases.
Pharmaceutical products have limited production runs, highly specific manufacturing requirements, and genotype-specific products, which has a direct impact from their supplier’s logistics and ensuring that the producer of these supplies are meeting FDA stringent quality requirements. This essential shift in these product mixes as well as the demand for continuous improvement in operation efficiency, greater quality control and higher visibility for track and traceability from “cradle to grave” in production has provided an evolution in the advancements in technologies and processes required for advanced Pharmaceutical manufacturing.
Numerous studies dedicated to Pharmaceutical manufacturing are focusing their investments in the following areas:
- To lower capital and operating costs; consistent quality improvement, and scalability should be executed based on continuous manufacturing improvement.
- New process analytical tools to improve material flow for greater visibility from suppliers to operations-process robustness, accelerating scale-up to commercial production and drive more efficient use of resources
- Single-use systems to increase flexibility and reduce production lead times, while lowering capital investment and energy requirements.
- Alternative upstream as well as downstream processing techniques, using analytics, material flow systems will greatly help to improve yields while lowering costs, and reducing waste, and improve energy management.


How an Industry Leader in Frozen Food Manufacturing used IoT, Big Data, and Machine Learning to Increase Yield.
Download the Case Study now and see how the ThinkIQ systems can help to expand your business!
What are the technology gaps in Pharmaceutical Manufacturing and what is needed?
Quality begins well before any material enters a pharmaceutical buildings operation. Quality begins at the source of the various suppliers and how quality is captured, tracked, and traced during the entire material(s) flow process through the suppliers’ operations. So how can we accomplish this large gap to insure a zero defect from cradle to grave? Challenge: Manual, tedious, and time-consuming data gathering resulting in a lack of process understanding and non-optimal processes. Solution: ThinkIQ for real-time material flow and QMS tracking of commercialization.
Results:
- End-to-end process understanding
- Reduced time hunting for data—now focused on producing, discovering and designing
- Reduction in material scrap, waste and rejected lots
- Significant annual savings in scrap avoidance depending on the stage of the process
- Improved quality and yield
- Faster time-to-market through shorter cycle times
- Increased profitability
- Improved energy management

What is Pharmaceutical Manufacturing?
The pharmaceutical industry is critical to human survival. Without medicine, many of the miracles of modern healthcare would not exist. It would follow that the most technically advanced manufacturing processes in the world drive pharma, right? Not necessarily. While the most advanced smart manufacturing technologies, often referred to as Industry 4.0, have been utilized in food & beverage, mining and metals, and more, it has yet to find widespread adoption in pharmaceutical factories.


The process behind Pharmaceutical Manufacturing.
Challenge: Manual processes leading to high costs and a lack of predictive capabilities.
All Pharmaceutical company goals in the manufacturing space is to achieve maximum product throughput with zero defects. To support this strategy manufacturing should consider starting with a continuous Improvement journey leveraging Lean and Six Sigma methodologies. Lean helps to ensure the required stability of manufacturing processes, eliminating waste, and allowing steps to flow smoothly with no interruptions, delays or bottlenecks for improved time to market. It also allows customers to “pull” the product as needed. Six Sigma helps to reduce variability using appropriate statistical tools for in-process control. Additionally, Design for Six Sigma can improve process robustness.

All these approaches rely on leveraging data generated across the organization. Data gathering is often cumbersome and heavily siloed for most company’s especially contract manufacturing Pharmaceutical companies. As data typically resides in paper-based documents, spreadsheets, and complex equipment systems often fail to provide it. For data to be useful, it is necessary to know what data is available, when it was created, its format, and whether it is qualitative or quantitative data. Gathering and formatting the needed analytics data from the material flow.
All too often the required information to make fast and better decisions are taking too long and become useless information. Operations are unable to garner insights from the data for impactful decision making, simply because this data was not provided in real time. Manually aggregated data sets typically represents for about 20% of the total parameters measured and the modeling most likely explain up to 60% of the results. These types of gaps must be directed in order to achieve the FDA requirements of Zero Defect based on Q10. Having a material flow solution that is capturing real time data building into a historian could accelerate data aggregation, modeling and prediction at the suppliers prior to entering into the Pharmaceutical companies and their contract manufacturers.
4 critical issues facing the pharma and healthcare industries
With the rising need for personalized drugs, smart manufacturing makes it possible for pharmaceutical factories to move away from batch manufacturing in which production is stopped for quality assurance testing. Each time production is stopped, lead times increase, and something can go wrong. With the flexibility, precision, and speed new technologies afford us, continuous manufacturing becomes possible by facing these 4 issues:
- With continuous manufacturing, substances move nonstop at a single facility, eliminating hold times, reducing the probability of human error and the likelihood of drug shortages.
- A more connected factory can better tap into emerging markets, compete with generics, and optimize its products.
- Greater visibility leads to globalization that can still align on a local level.
- Production times can be slashed with access to real-time information.


Why is supply chain traceability important and specifically for pharmaceutical safety?
Pharmaceuticals are closely and extensively regulated, of course. So much so, it can be difficult to adopt new technologies. Such change, after all, would likely lead to process revalidation, an undesirable initial result. Plants use specific kinds of equipment that require meticulous inspection, their processes validated in scrupulous detail. Despite the hurdles, smart manufacturing offers incredible upside in terms of supply chain traceability. Upside that is well worth it.
Prior generations of breakthroughs in manufacturing were centered more on the shift from mechanical technology to digital computing and communications. This current shift takes those advances a step further by adding autonomy through artificial intelligence (AI), machine learning (ML), and the internet of things (IoT). In other words, we’re now endeavoring not just to produce massive amounts of data but to digest it into smart decision-making. These autonomous systems seek to improve manufacturing processes and quality assurance to increase productivity, efficiency, and quality while reducing risk and adhering to strict regulations.

What are recent renovations in pharmaceutical supply chain safety?
With ThinkIQ’s Transformational Intelligence platform, pharmaceutical manufacturers gain access to a complete overview of all operations. From lab to jab, our smart manufacturing technology, including our semantic model and material ledger, dramatically reduces recalls, identifies weaknesses in the plant, and eliminates safety concerns.
A material ledger provides an innovative approach to intelligent tracking of material, and energy movement and transformations, their associate monetary value, and quality data. It delivers insight that improves yield, quality, safety, compliance, and brand confidence through a fact-based granular, data-centric contextualized view of material flows and related provenance attribute data. We utilize existing IoT infrastructure on which a cyber-physical system can be built to decentralize decision-making in favor of autonomous task-completion of all but the highest-level exceptions. It’s the next generation of smart manufacturing for the most mission-critical industry.


How is pharmaceutical manufacturing technology affecting the Healthcare and Medical Industries?
Competition, unexpected events, and consumer demand put relentless pressure on manufacturers. No manufacturing company can succeed over the long term unless their costs are below the average of all competitors offering equivalent products or services to the same customer segments. Short-term survival might be possible but not long-term success. Cost reductions do not come automatically, they required constant management attentiveness in all matters to insure productivity gains and cost reductions. Too often products and costs drift out of competitive line, and no one realizes it until it is too late. Greater visibility in real time of the material flow prior to entering the process operations provides most all the tools to achieve zero defect, effectively manage your cost of production, from BOM Management, to Material Requirements Planning, to Capacity Requirements Planning, to Shop Floor Control, and Product Quality. Whether your business is discrete or process, make to stock or make to order, or both, material flow solutions will improve efficiencies visibility, and quality across your entire operation.
The FDA has approved continuous process manufacturing since 2015, including for products made by Janssen, Eli Lilly, and Pfizer. The FDA has cited many positive advantages of continuous processing:
- The elimination of manual handling and human error
- Increased quality assurance through online monitoring and control
- Reduced manufacturing time and increased efficiency
- Reduced capital costs utilizing smaller equipment and less manufacturing space
- More nimble responsiveness in the event of a drug shortage
- More tailored drug production to fit the needs of precision medicine
Manufacturers also enjoy greater utilization, more flexible batch sizes, simplified scaling, more control over critical process parameters, less energy consumption, and more reliable scheduling.

Why Artificial intelligence is helping pharma manufacturers define and pursue next level security and developments in the global regulatory environment.
In pharmaceutical manufacturing, patience is no longer a virtue. With the rise of personalized medicine and swift changes in consumer demand, batch processing is becoming less viable. Considering increased competition, rising costs, and pricing pressure, product revenues have declined to the point that the same technologies the industry has relied on for decades are finally showing their limitations. The use of artificial intelligence is facilitating new levels of visibility and understanding of ingredients as they flow across the supply chain.


What is the Roadmap to Becoming a Smart Manufacturing Firm?
ThinkIQ’s Transformational Intelligence platform, can provide your pharmaceutical operation a complete overview of all manufacturing processes. Our in-depth, industry-specific experience with supply chain and manufacturing concerns help manufacturers avoid recalls from temperature spikes and assembly-line slow down, and spot weaknesses in product that could become a major safety concern. By identifying and executing even a small change in raw ingredients or suppliers can significantly increase your bottom line. We use industry benchmarking and KPIs to surface the manufacturing issues that matter across plants, across supply chains, and around the world.

“We reply upon the manufacturing controls and standards to ensure that time and time again, lot after lot, year after year, the same clinical profile will be delivered because the product will be the same in its quality. We have to think of the primary customers as people consuming that medicine and we have to think of the statute and what we are guaranteeing in there: that the drug will continue to be safe and effective, and perform as described on the label.” – Janet Woodcock, M.D.
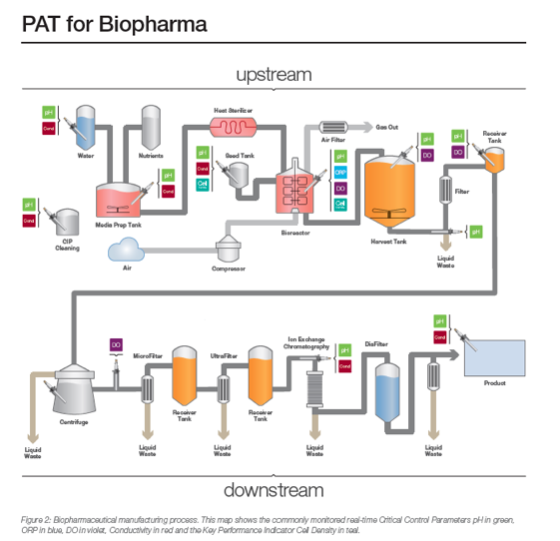
U.S. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research (CDER), Center for Biologics Evaluation and Research (CBER) .
Background Reference: Q10 Pharmaceutical Quality System
- Foundation: Regional GMP (drug product) requirements, the ICH guidance “Q7 Good Manufacturing Practice Guidance for
- Active Pharmaceutical Ingredients,” and ISO quality management system guidelines form the foundation for ICH Q10.
- Harmonization: ICH Q10 provides a harmonized model for a PQS.
- Lifecycle: Defines how a modern quality system assures science- and risk-based drug manufacturing and quality decisions throughout the lifecycle.